Owner:
Integra IT
Year:
2023
Skills:
Generative & Evaluative Research
Information Architecture
Usability testing
Interface Design
Interaction Design
We collaborated with the entire team to improve the user experience, interface, and interaction of the feature for reading and signing informed consent in clinical trials.
We reduced the time researchers spent on participant assignment and follow-up by 62%, while increasing participant comprehension and satisfaction by 85% when reading and signing the consent form.
Owner:
Integra IT
Year:
2023
Skills:
Generative & Evaluative Research
Information Architecture
Usability testing
Interface Design
Interaction Design





Integra IT provides a range of tools that ensure the quality and transparency of data reported in clinical trials for new vaccines, all while complying with legal regulations.Initially, the company focused on data execution, monitoring, and reconciliation.
However, to help research centers centralize information and reduce delays in meeting trial schedules, we developed a solution for participant recruitment and assignment, as well as the explanation and signing of informed consent documents.
And the team said:
"...the tool is almost fully designed; we just need a few more screens."
When I joined the Integra IT team, my first challenge was working on the module for setting up, reading, and signing the informed consent document.
The project had started a few months earlier, and while progress had been made on several modules, it stalled due to limited development capacity and other business priorities.
I quickly identified a few initial issues:
The user contexts weren't analyzed, and the flow was built solely on internal team assumptions.
The solution had issues in navigation, visuals, and the content within the flows already developed by the team.
Components from various libraries were used, but many didn't fit the needs of the problem we were trying to solve.
The first thing I did was discuss with key stakeholders the risks of continuing development without properly understanding and analyzing the created flows, and without engaging the users involved in testing the tool.
After this meeting, we agreed to carry out the following activities to identify improvement opportunities and user needs. These would guide our decisions and help us prioritize for the MVP launch of the solution:

UX audit of the initial flow, guided by Jakob Nielsen’s 10 usability heuristics.

6 interviews with clinical trial participants to understand the recruitment process and informed consent signing.

8 flow tests and interviews with research center professionals (doctors and recruiters).
Most of the issues we observed stem from people being used to a rudimentary process, where they spend a lot of time working with multiple tools to complete tasks.

- Clinical trial participants have diverse cultural, social, and economic needs, which makes initial understanding and learning challenging.
- 66% of participants primarily use slow, outdated mobile devices and lack access to computers.
- 83% of this group do not have mobile data plans or home internet; they rely on prepaid data packages when needed.

- Missing key data in forms to meet legal requirements regarding the number of signatures needed, based on the country where the clinical study is conducted.
- 75% of users did not complete the setup and assignment of informed consent, citing the process as tedious and lacking clear guidance on the steps.
- The main challenge for research centers is the time spent explaining the study to participants, which typically takes 45 minutes per person.
- Informed consent forms vary in certain details between different clinical trials within the same research center.

- The forms lack interactions to indicate required fields and potential errors that could occur when using the tool.
- The language in the flows is unclear, with little guidance on tasks, lacking content that instills confidence in the user.
- No section was provided to help users navigate the setup process or track the status of the informed consent.

After sharing all the findings with the team, we posed the following questions to answer, which helped us define the objective and key metrics.
How might we provide an effective tool that takes into account the technological limitations of the participants?
How might we prevent recruitment times from extending, ensuring we stay on track with the clinical trial schedule?
How might we ensure participants fully understand the clinical trial before their first appointment with the investigator?
Reduce the time researchers currently spend with participants on understanding the clinical study by 75%.
Decrease the percentage of delays in the execution of the clinical study timeline by 60%.
Ensure that 100% of participants complete the study comprehension process before their first medical appointment.
Increase the number of active sessions by research center staff by 55%.


After negotiating the scope of improvements with the team for certain stages of the process and testing with the identified user segments, we handed over the following features for the informed consent tool to the development team.

As a member of the IntegraIT support team, I want to accurately configure the client's clinical study information and have a complete overview to assist them.
This stage will be handled by the Integra IT support team, where they will be provided with the clinical study protocol, and the support member will create the informed consent based on the current stage of the study.




At this stage of the process, the person responsible for the setup will need to take the informed consent document and create each chapter, which will be displayed in the app used by participants. They can include videos, images, or links, and will also create evaluation questions for participants, guardians, or witnesses.


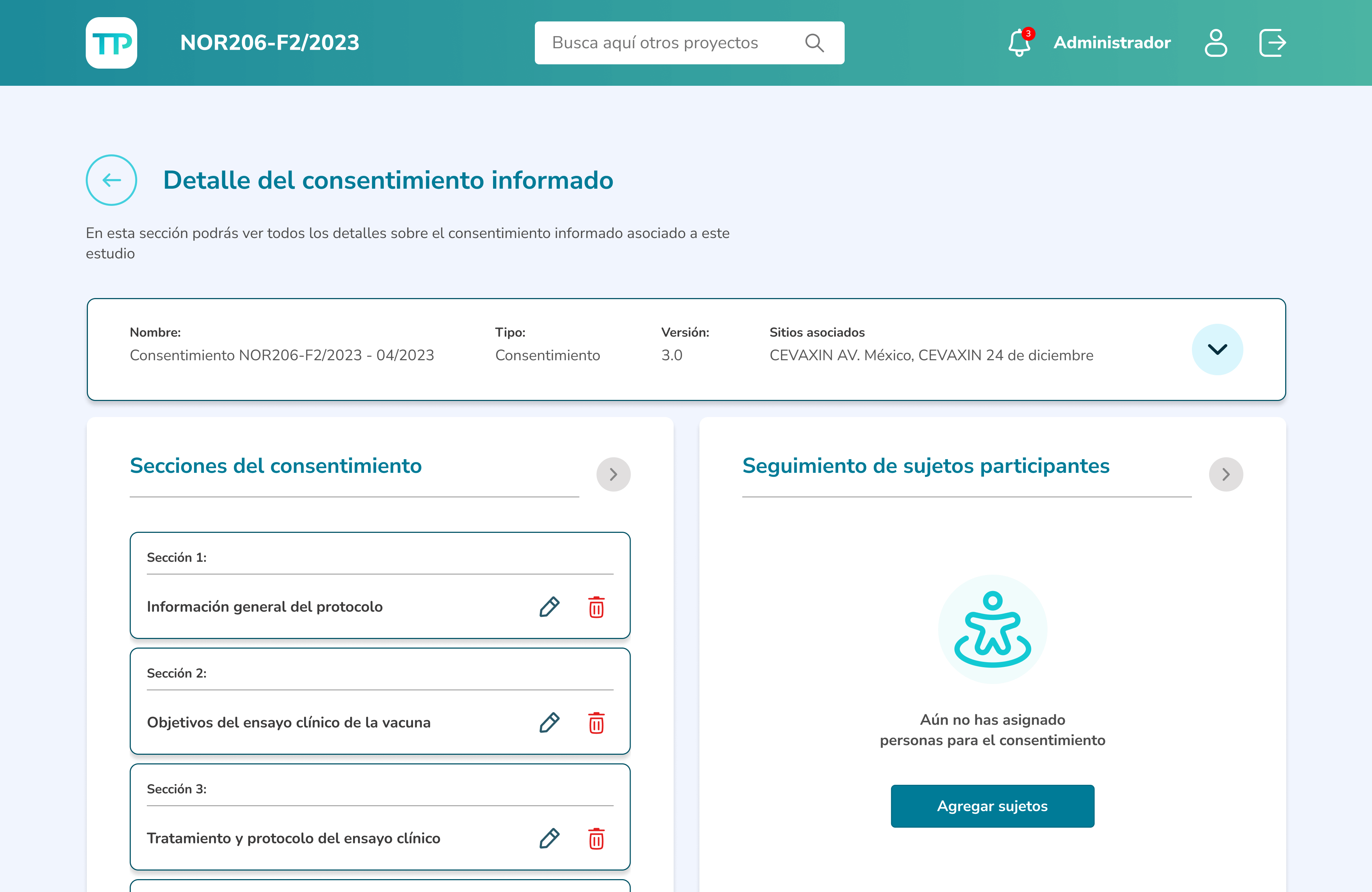

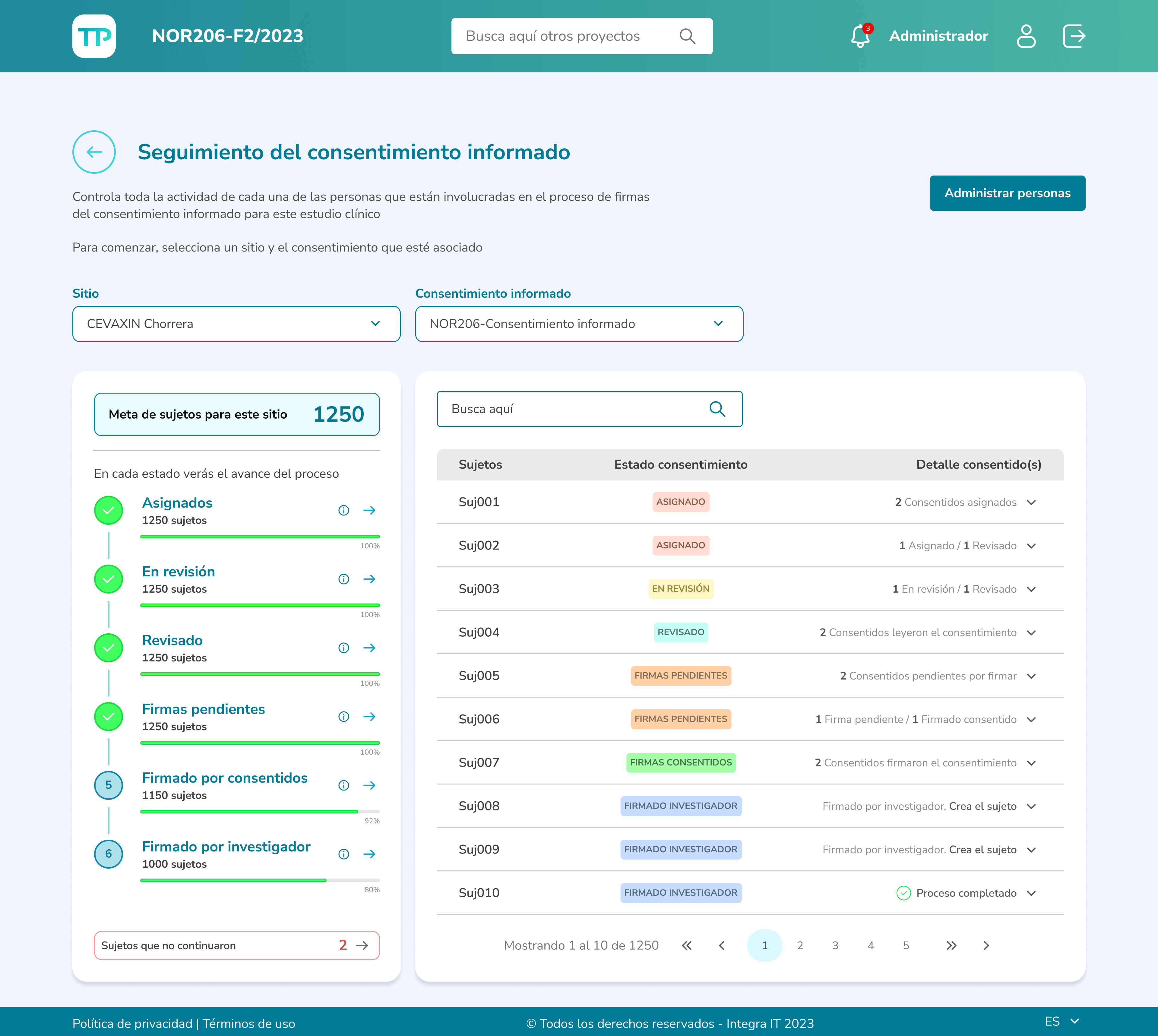
As a clinical doctor and researcher, I want to have clear tracking of participant activity and ensure a better understanding of the clinical study.
Those involved in the research process can assign people as participants either manually through the form or by uploading a CSV file.

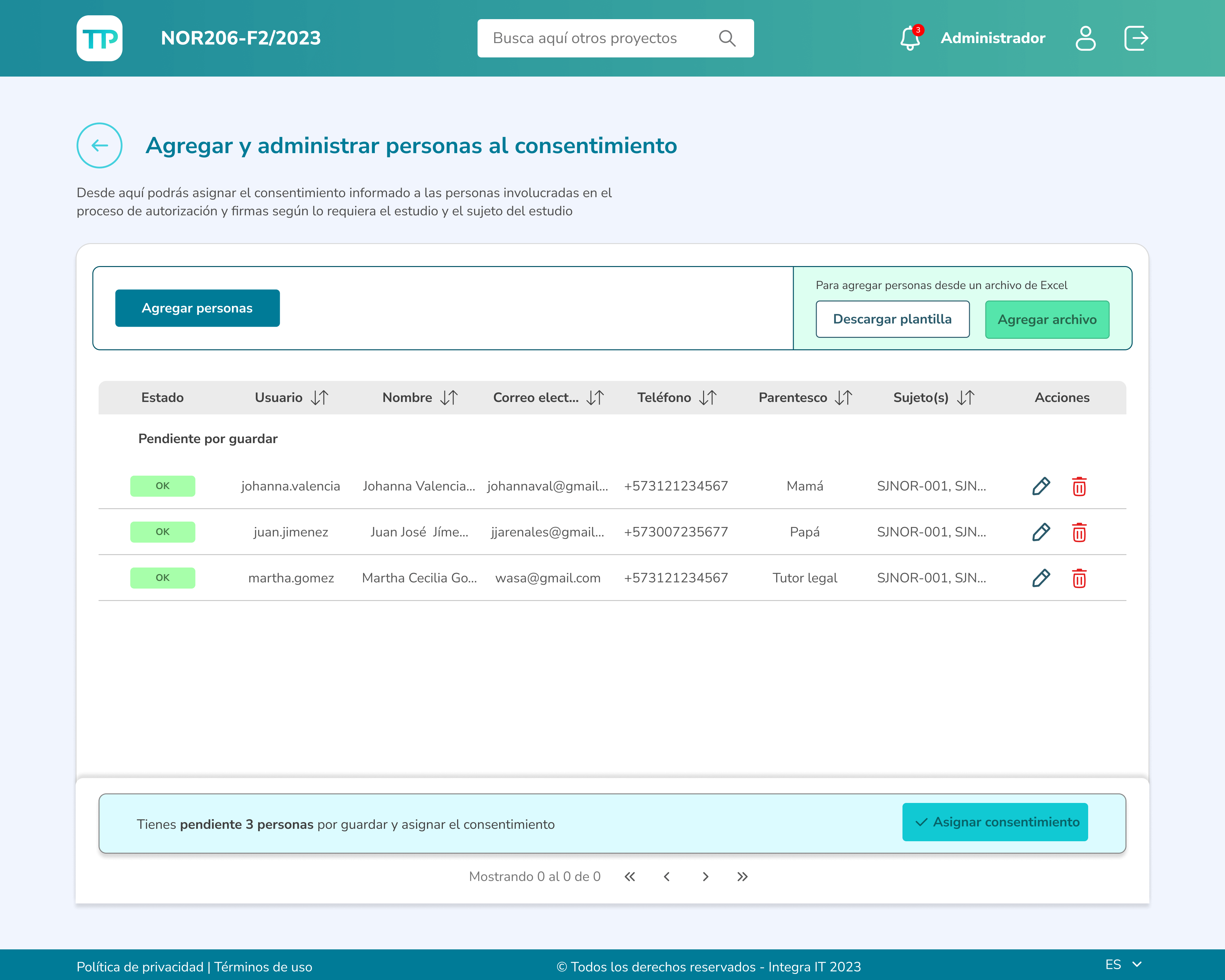
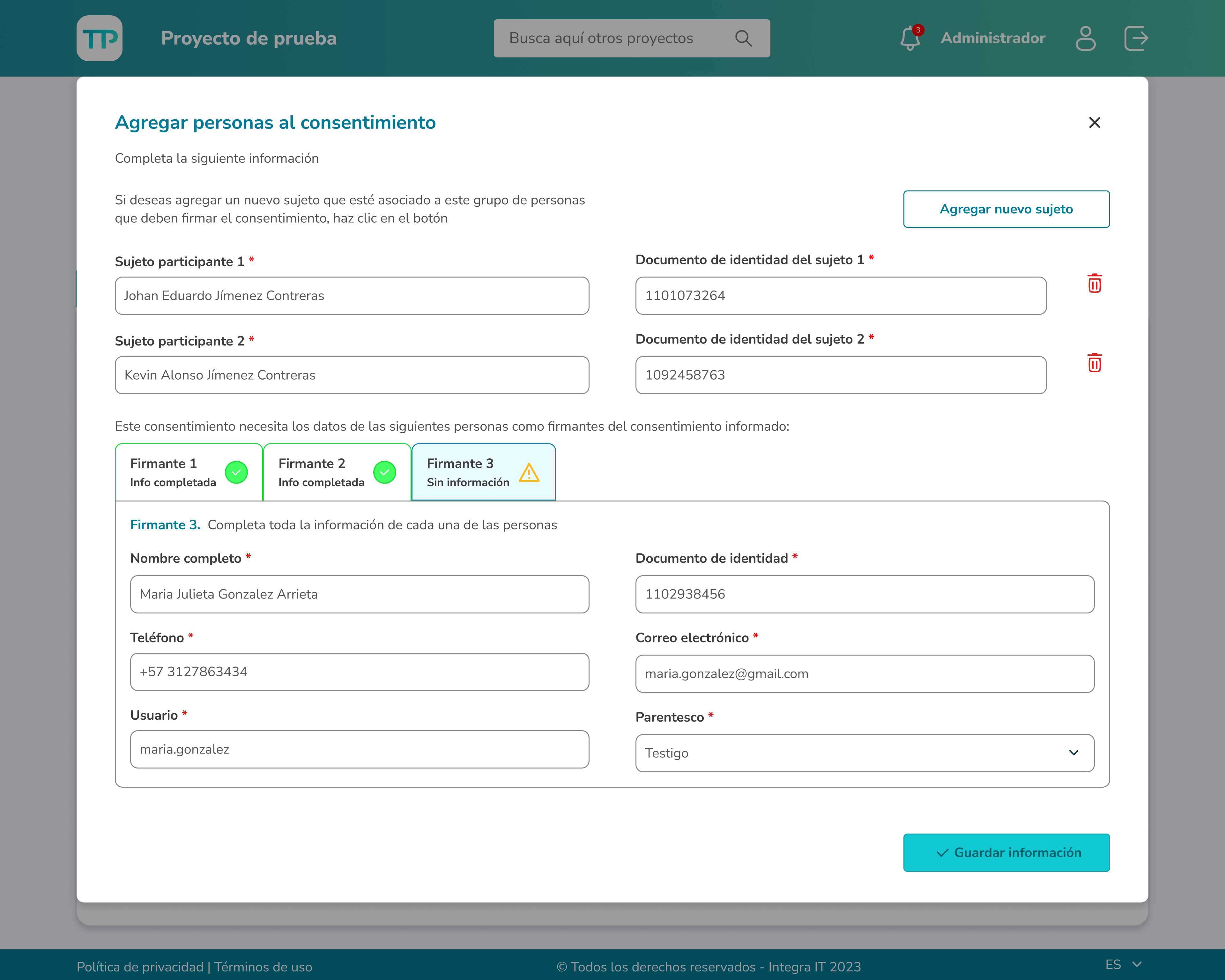
Clinical investigators can monitor participants' reading progress and have access to various actions, such as reviewing reading times, evaluating their answers to the assessment questions, and enabling participants to sign the document.




As a clinical trial participant, I want to quickly understand what the clinical study will involve and avoid spending too much time at the medical center.
Participants, guardians, and witnesses involved in the informed consent signing process will need to read and answer a set of questions that will assess their understanding of the clinical study before the first appointment with the medical research team.




After taking the initial solution proposed for the MVP and conducting a thorough understanding of the users' pain points, we achieved the following:
- We reduced the time a clinical investigator typically spends explaining the study to participants by 62%, cutting it down from 45 minutes to around 17 to 20 minutes during the first appointment.
- We improved typography styles and interaction patterns, initiating a technical refinement of the component and style libraries. This was aimed at unifying the visual language and optimizing performance.
- Based on the test results, as well as technical and time constraints, we adjusted the MVP experience by having the configuration handled by Integra IT. This involved assigning a dedicated support team member to each client. We also committed to analyzing and improving the configuration process before launching it to clients.